ITP is characterized by low platelet counts and symptoms like bleeding and bruising1
ITP is an acquired autoimmune disorder characterized by low platelet counts that result from both increased platelet destruction and/or impaired platelet production.2-4
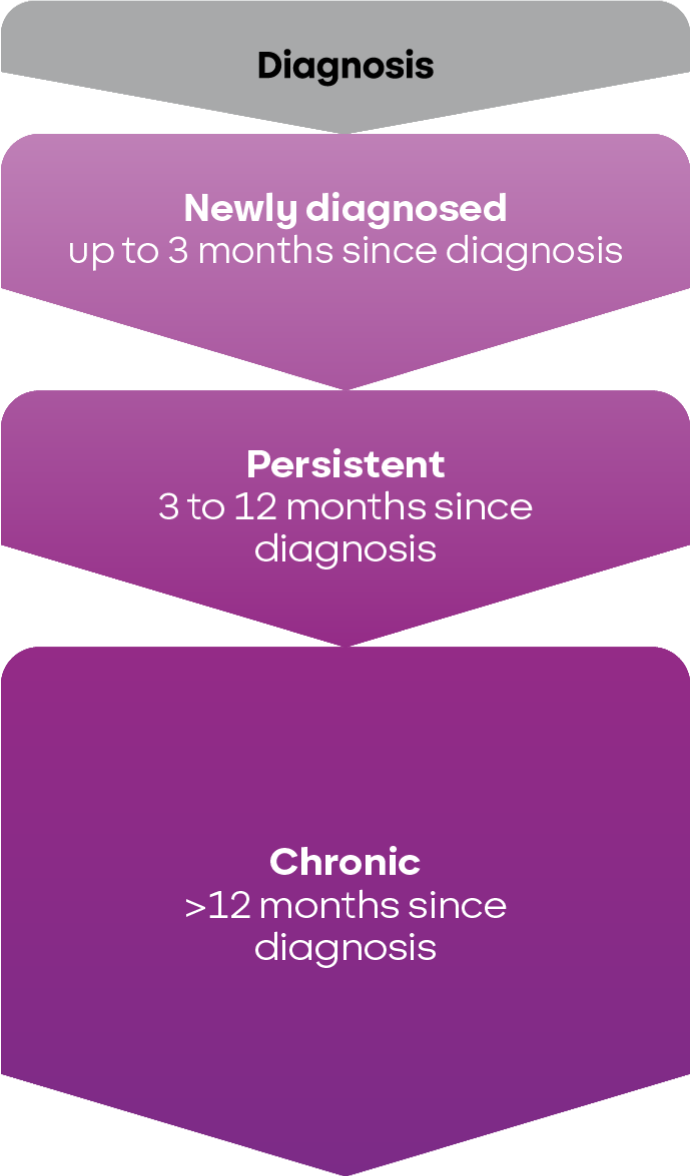
The time since diagnosis helps classify ITP as newly diagnosed, persistent, or chronic.5
Multiple symptoms of ITP are related to bleeding and can include6-10:

Petechiae and purpura, usually on the extremities
%201.png)
Bleeding blisters
and epistaxis

Organ bleeding
(eg, menorrhagia)

Intracranial
haemorrhage
In some studies, severe bleeding occurred in approximately 7% to 10% of adult patients and typically correlated with lower platelet counts.11,12*
*
As seen in a cross-sectional study of 302 French patients, as well as in a systematic review of 147 clinical studies of adults and children with primary ITP.11,12
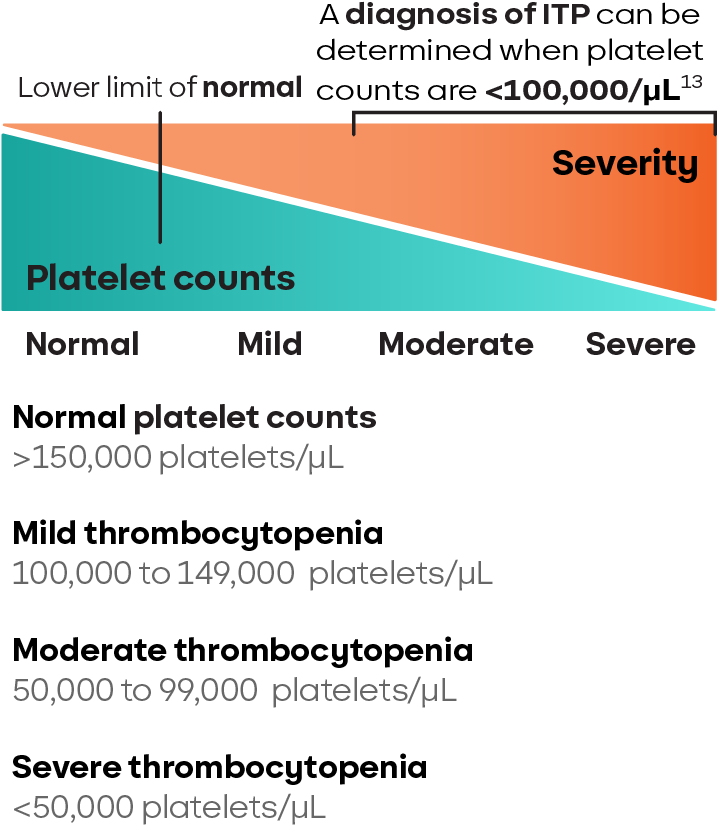
While platelet counts below 100,000/μL can indicate ITP, the lack of a specific diagnostic test means other potential causes of thrombocytopenia must be ruled out.2
It is also important to determine if ITP is primary or secondary to another condition. There are multiple causes of secondary ITP, including chronic lymphocytic leukemia (CLL), Helicobacter pylori infection, and COVID-19 infection.2,14
ITP can occur in people of all ages. The incidence of ITP ranges from 2 to 4 cases per 100,000 person-years in adults and children.2,15
The prevalence of ITP is estimated to be 58 per 100,000 adults and between 4.1 and 9.3 per 100,000 children (ages 1 to 18 years).16,17
The pathophysiology of ITP is complex and not fully understood2
In ITP, autoreactive B cells produce autoantibodies that target platelets for destruction by macrophages and impair megakaryocyte maturation, which then inhibits platelet production. Autoantibodies may also destroy platelets through other mechanisms. Cytotoxic T cells can also directly destroy or inhibit the production of platelets.2
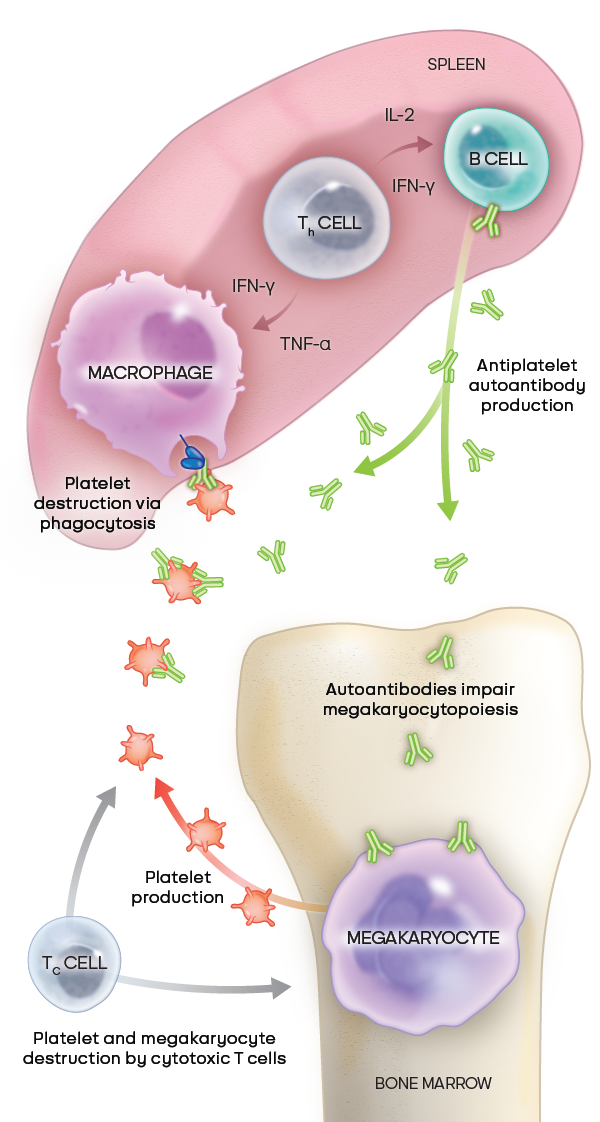
Adapted from Kashiwagi H, et al. Int J Hematol. 2013;98(1):24-33. © Japanese Society of Hematology (JSH).18
Bruton’s tyrosine kinase (BTK) drives key processes in B cells and macrophages19,20
B cells are critical to autoantibody production and macrophages are critical to phagocytosis. Both of these processes are regulated by BTK.
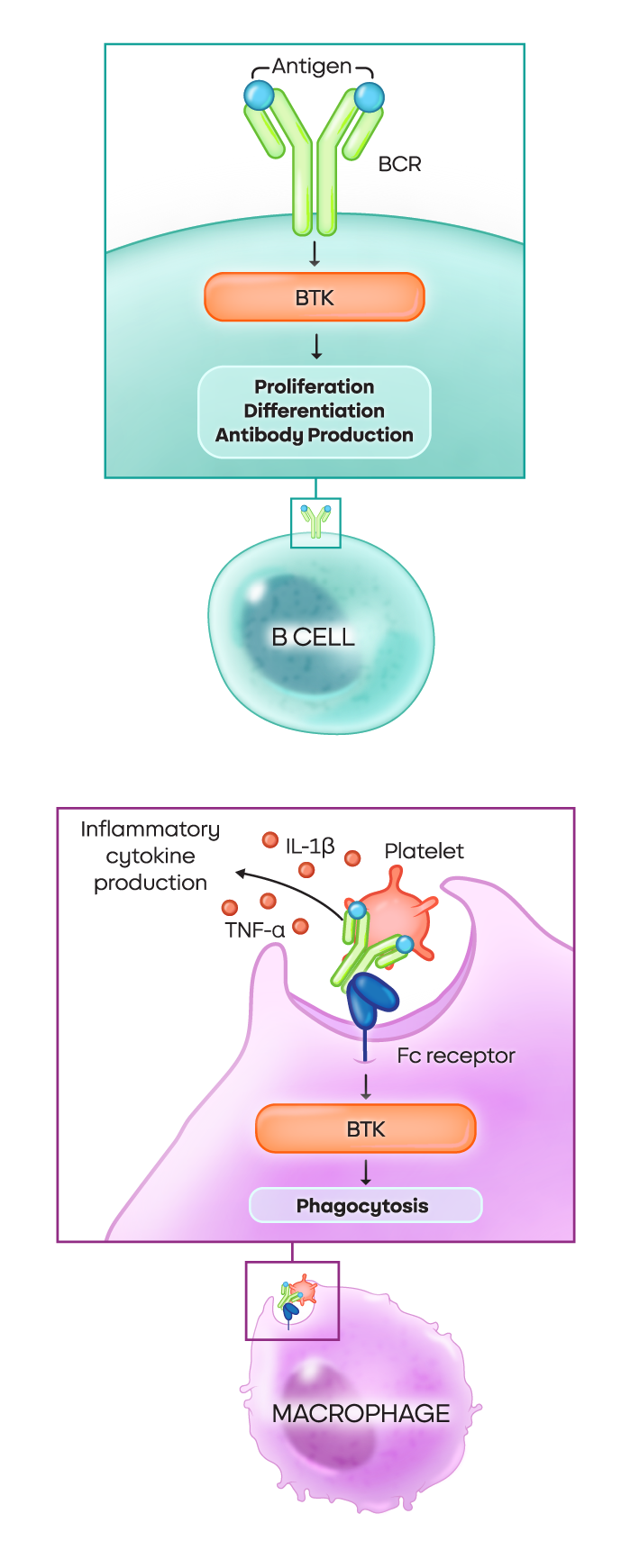
- In B cells: The BTK pathway is crucial for proliferation, differentiation, and autoantibody production
- In macrophages: Various signaling pathways dependent on BTK drive phagocytosis, degranulation, and the production of inflammatory cytokines
BTK is also a critical driver of inflammation20
BTK plays a key role in the production or activation of these inflammatory markers:
• TNF-α
• IL-6
• NLRP3 inflammasome
• IFN-γ
Next: ITP is about more than platelets
Learn how ITP symptoms like fatigue and cognitive impairment impact patients’ quality of life.
1. Rovó A, Cantoni N, Samii K, et al. Real-world impact of primary immune thrombocytopenia and treatment with thrombopoietin receptor agonists on quality of life based on patient-reported experience: Results from a questionnaire conducted in Switzerland, Austria, and Belgium. PLoS One. 2022;17(4):e0267342. 2. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945-955. 3. Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol. 2021;96(2):199-207. 4. Mitchell E, Frith J, Newton J. Fatigue and cognitive impairment in immune thrombocytopenic purpura remain stable over time: short report from a longitudinal study. Br J Haematol. 2019;186(5):777-781. 5. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. 6. Kohli R, Chaturvedi S. Epidemiology and clinical manifestations of immune thrombocytopenia. Hämostaseologie. 2019;39(3):238-249. doi:10.1055/s-0039-1683416 7. Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495-520. doi:10.1016/j.hoc.2013.03.001 8. Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121(14):2596-2606. doi: 10.1182/blood-2012-07-442392 9. Cooper N, Morrison MA, Vladescu C, et al. Identification of occult cerebral microbleeds in adults with immune thrombocytopenia. Blood. 2020;136(25):2875-2880. 10. van Dijk WEM, Nap-van der Vlist MM, Knoop H, Schutgens REG. Possible targets to reduce fatigue in chronic immune thrombocytopenia patients – an explorative study. TH Open. 2022;6(4):e387-e395. 11. Piel-Julian M-L, Mahévas M, Germain J, et al; CARMEN Investigators Group. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost. 2018;16(9):1830-1842. 12. Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457-464. 13. Williamson DR, Albert M, Heels-Ansdell D, et al; PROTECT collaborators, the Canadian Critical Care Trials Group, and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144(4):1207-1215. doi:10.1378/chest.13-0121 14. Kewan T, Gunaratne TN, Mushtaq K, et al. Outcomes and management of immune thrombocytopenia secondary to COVID-19: Cleveland clinic experience. Transfusion. 2021;61(7):2014-2018. doi:10.1111/trf.16368 15. Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235-244. 16. Segal JB, Powe JB. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4(11):2377-2383. 17. Sanofi Genzyme. Data on file. 18. Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98(1):24-33. © Japanese Society of Hematology (JSH). 19. Zhu S, Gokhale S, Jung J, et al. Multifaceted immunomodulatory effects of the BTK inhibitors ibrutinib and acalabrutinib on different immune cell subsets – beyond B lymphocytes. Front Cell Dev Biol. 2021;9:727531. 20. Neys SFH, Hendriks RW, Corneth OBJ. Targeting Bruton’s tyrosine kinase in inflammatory and autoimmune pathologies. Front Cell Dev Biol. 2021;9:668131.